Purpose of the Laboratory Tests
As part of this long-term investigation, laboratory testing was used as a grounding tool — a way to keep the work anchored in measurable physiology rather than interpretation alone.
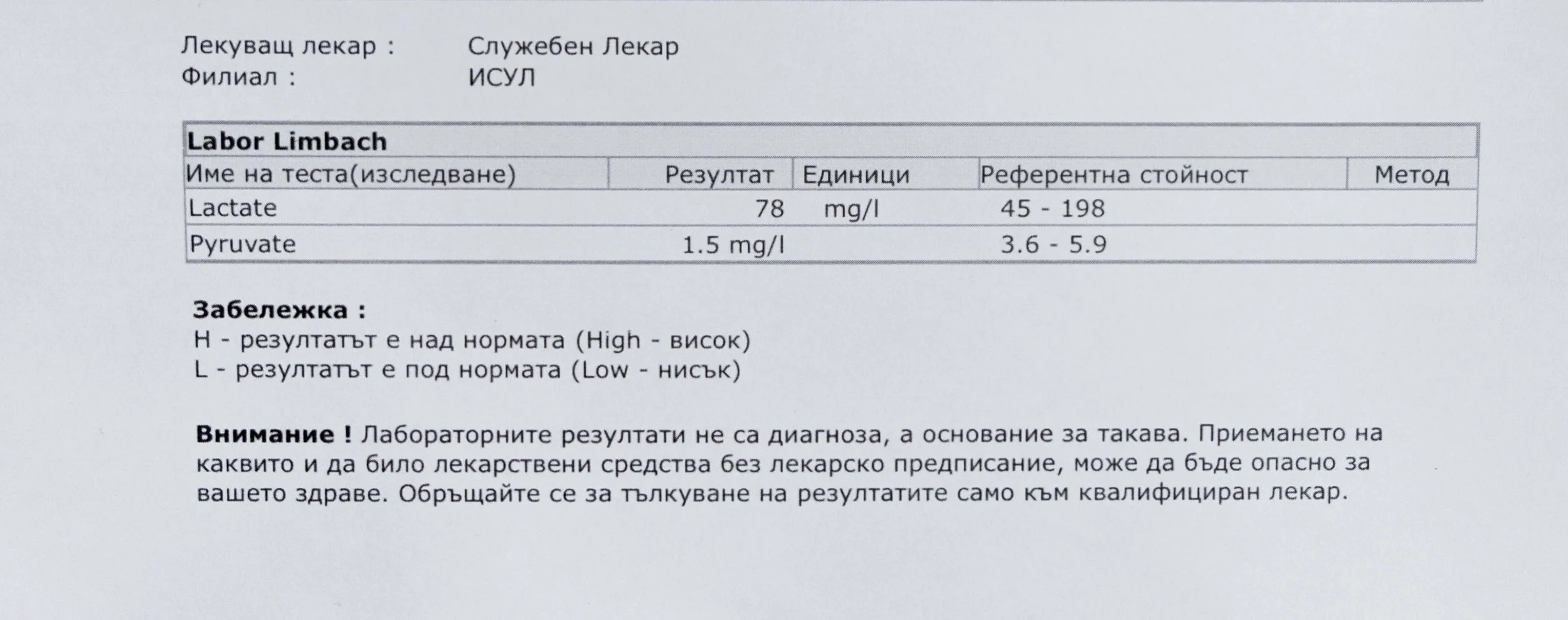
The research began with prolonged exposure to common modern stimulants and substances, including nicotine, caffeine, cannabis, sugar, alcohol, and, more recently, petroleum-derived compounds. Although chemically different, these substances share a common feature: they introduce repeated methyl-group activity into human metabolism. Over time, this alters how energy, memory, and signaling are processed in the body.
Rather than treating these effects as psychological or behavioral, the research followed their biological footprint.
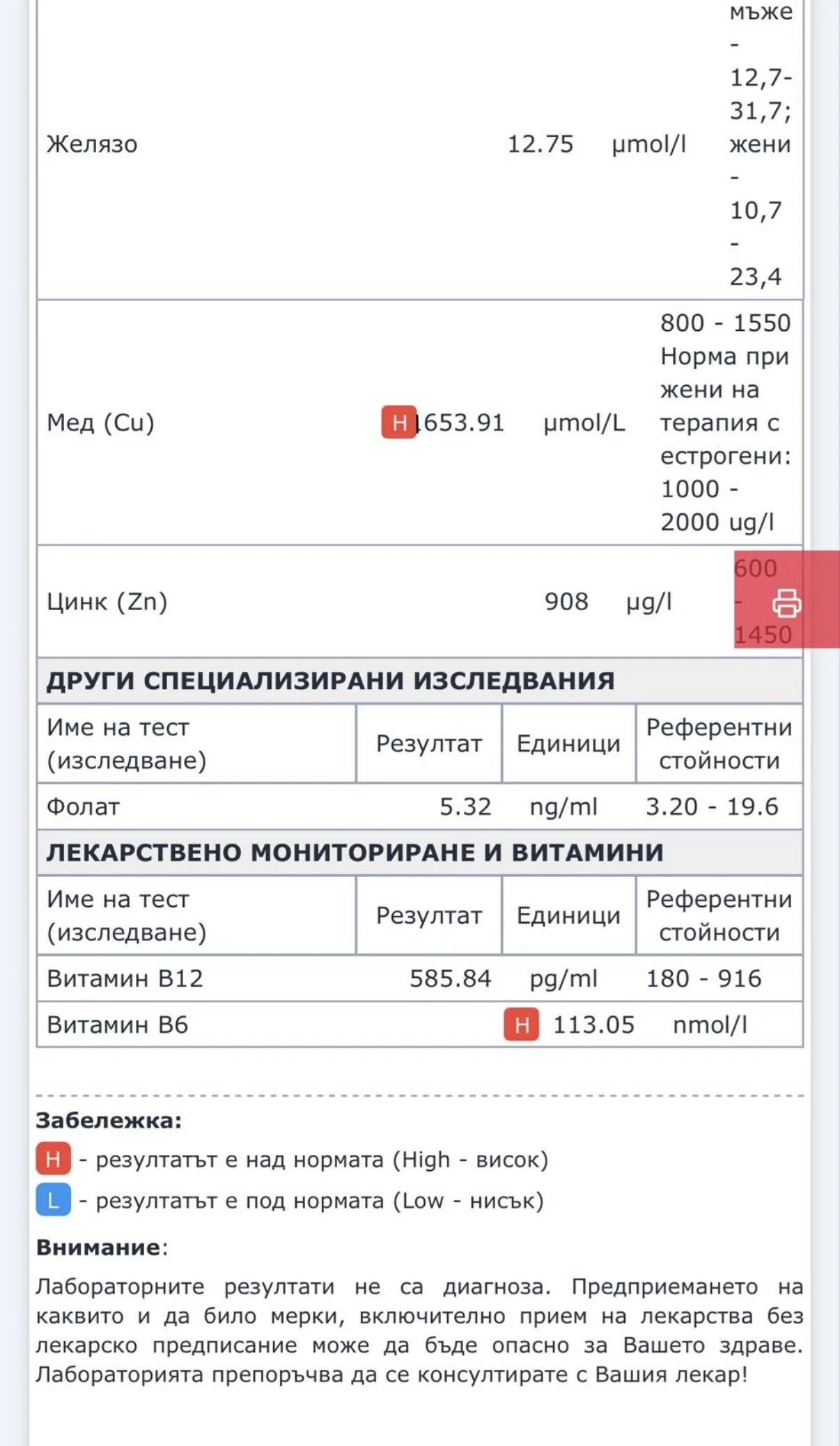
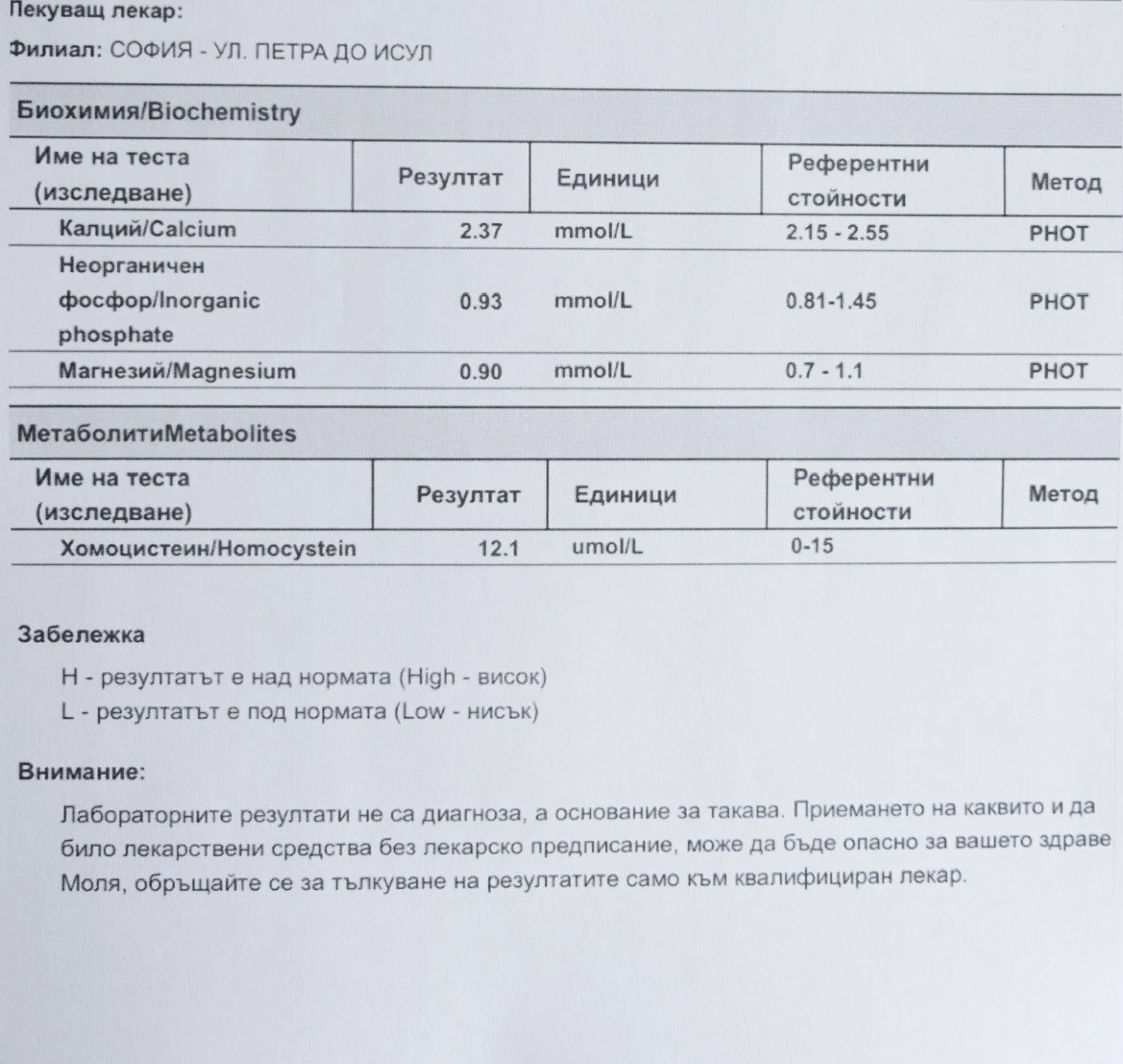
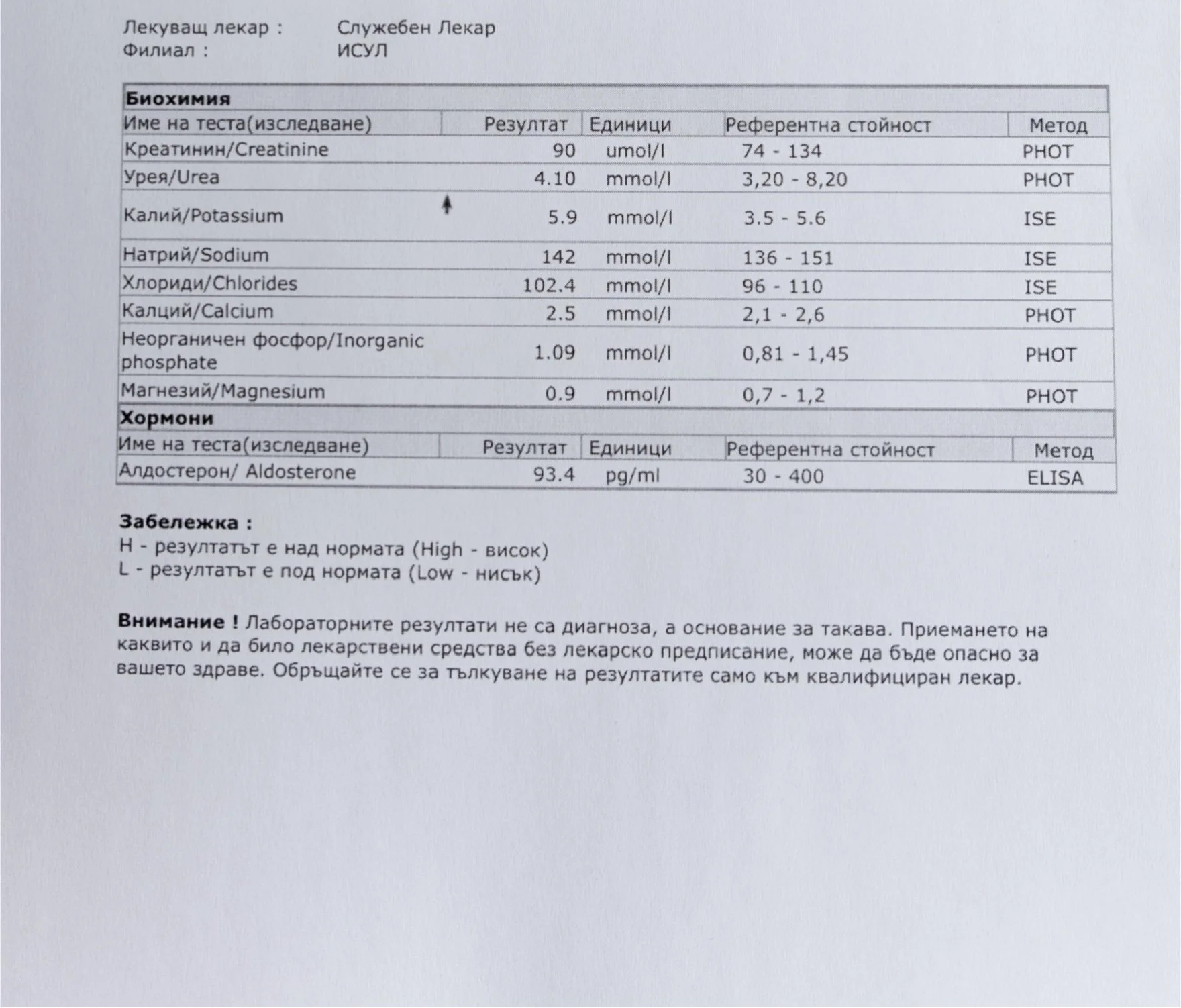
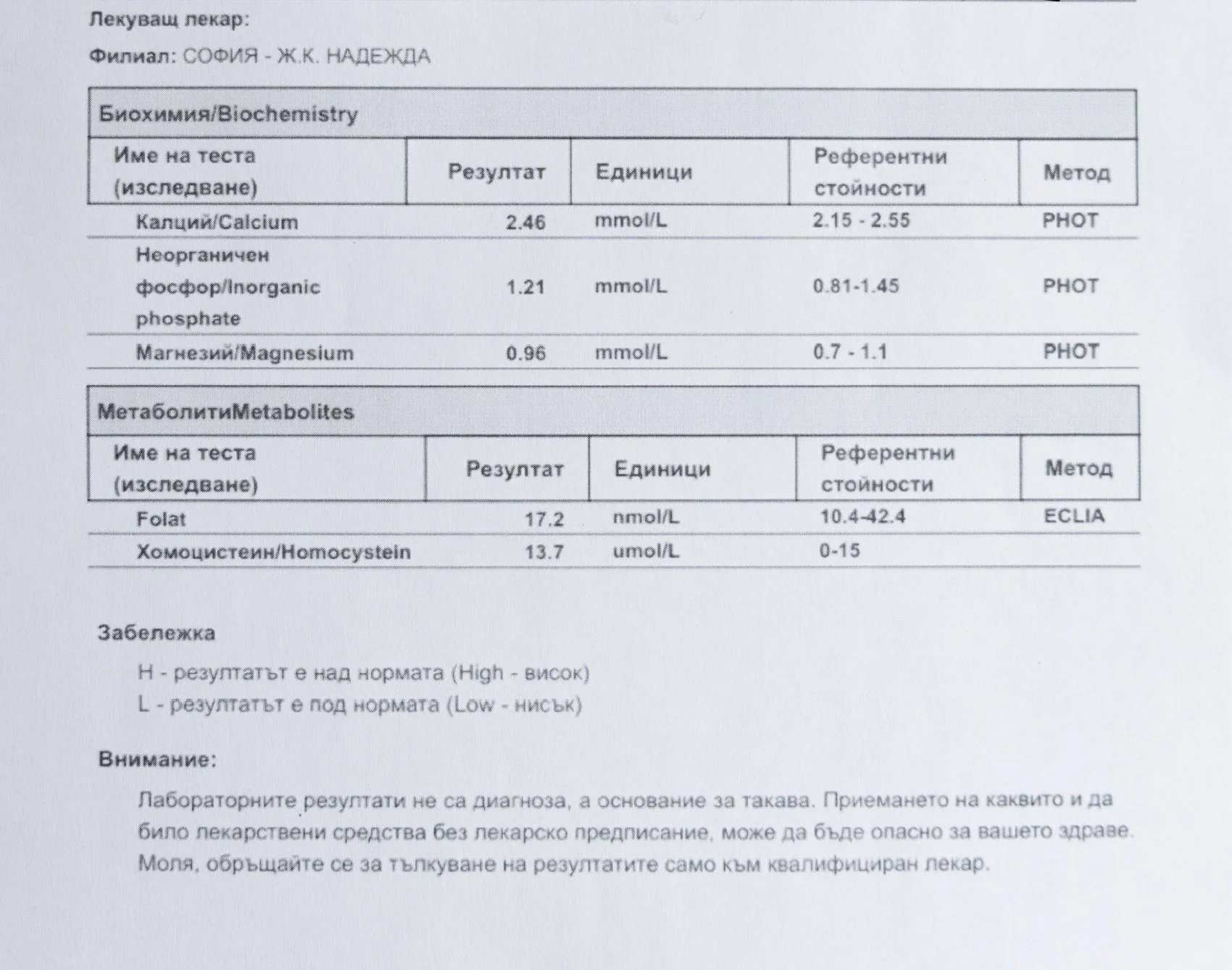
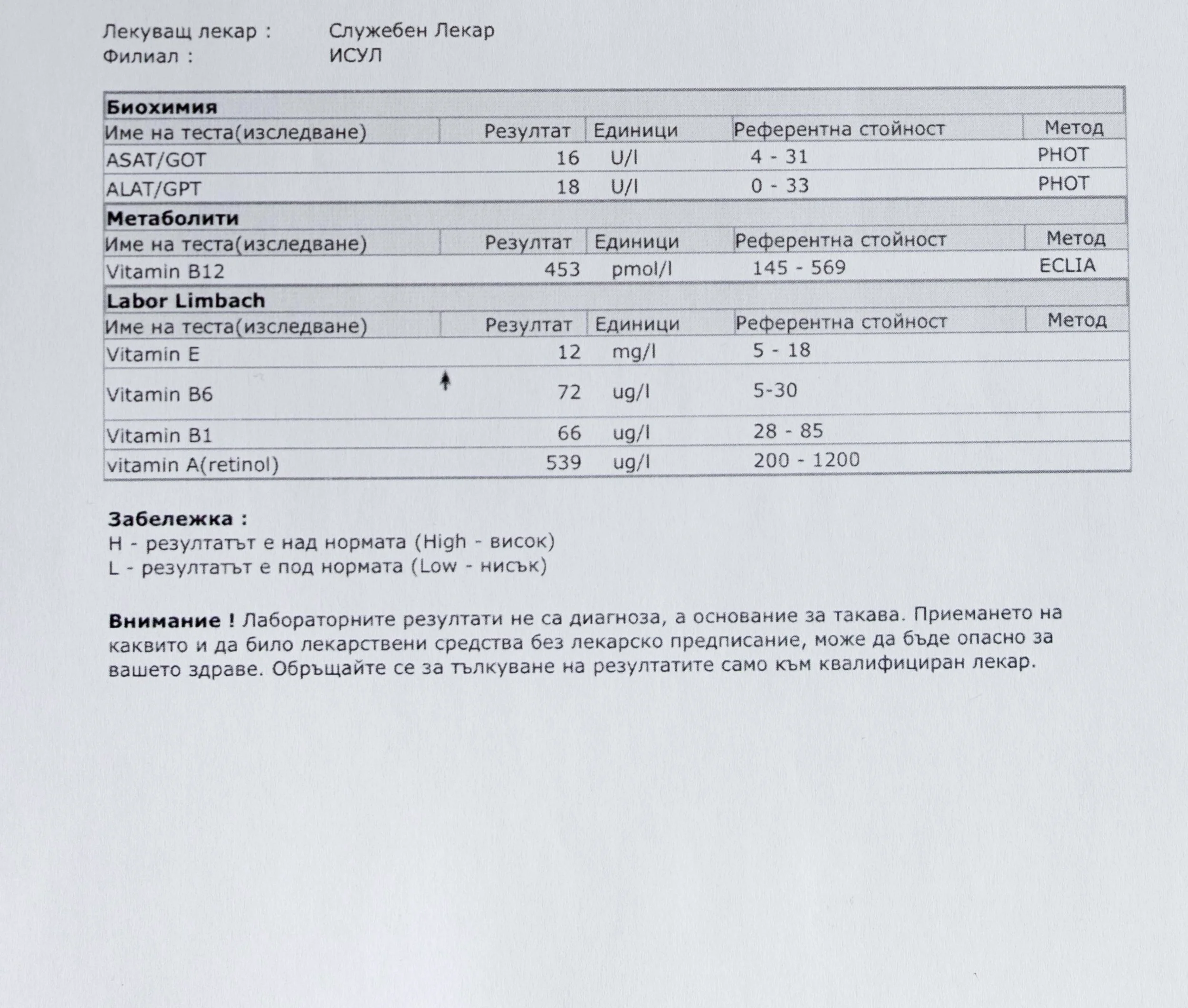
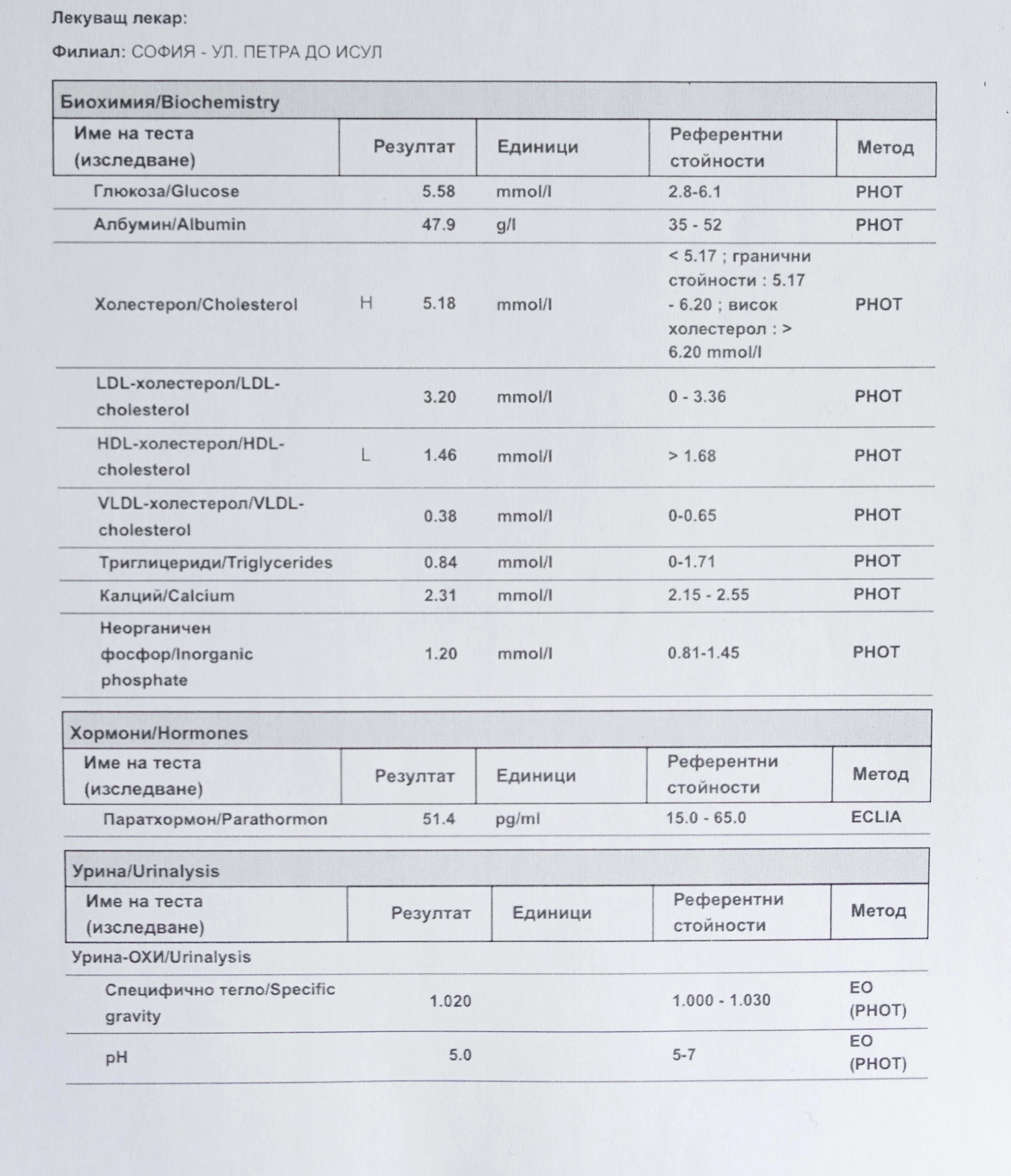
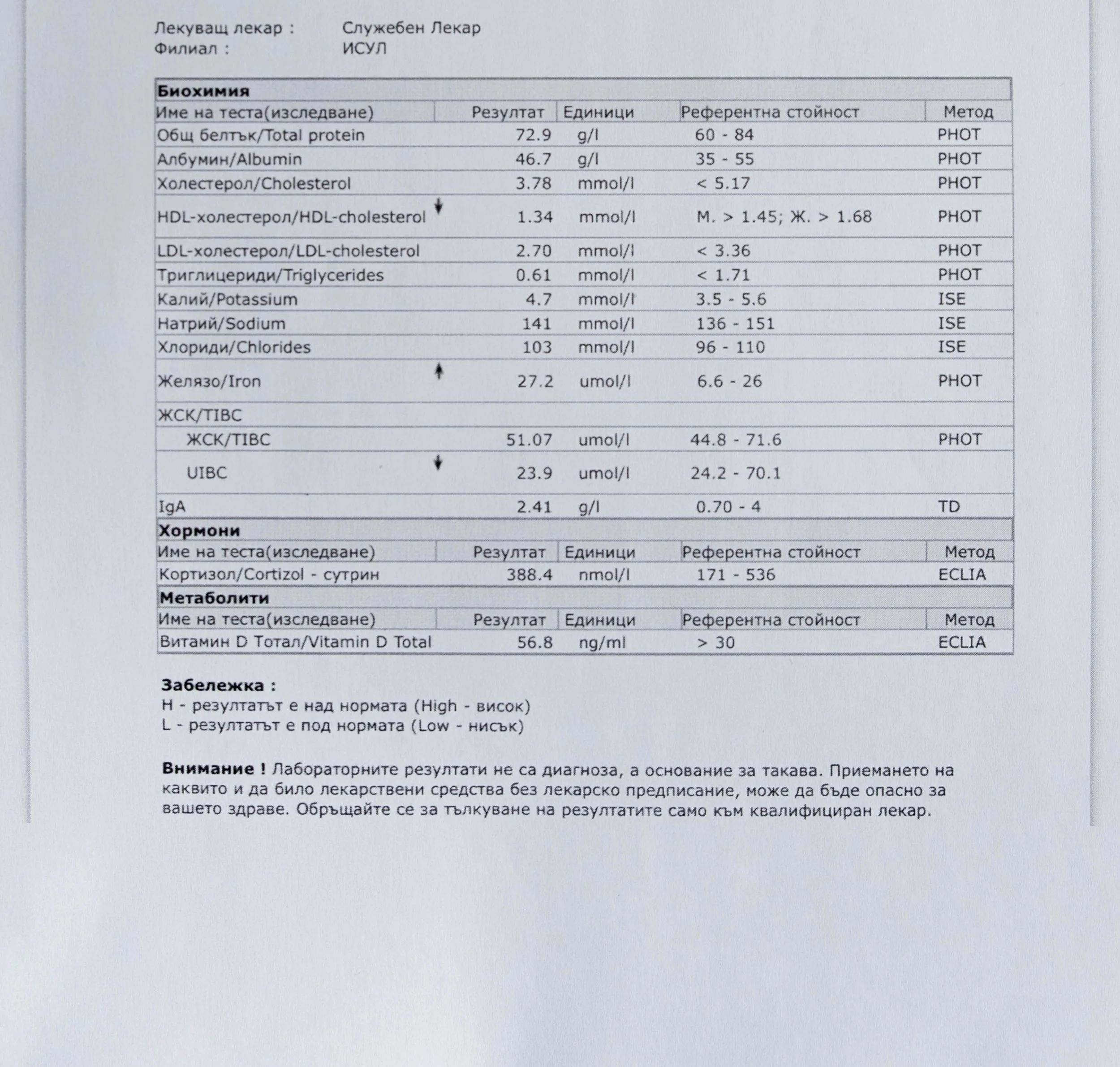
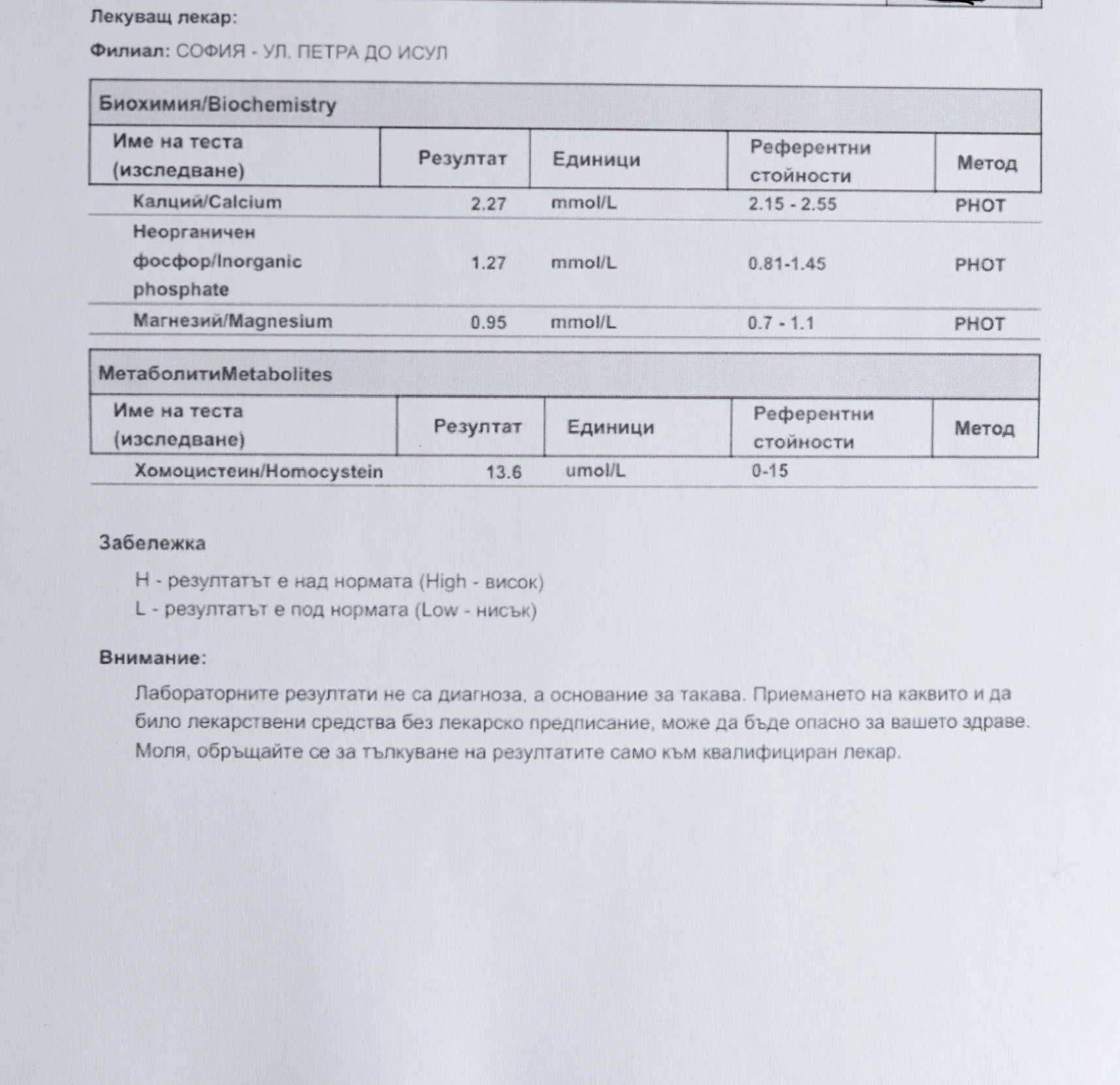
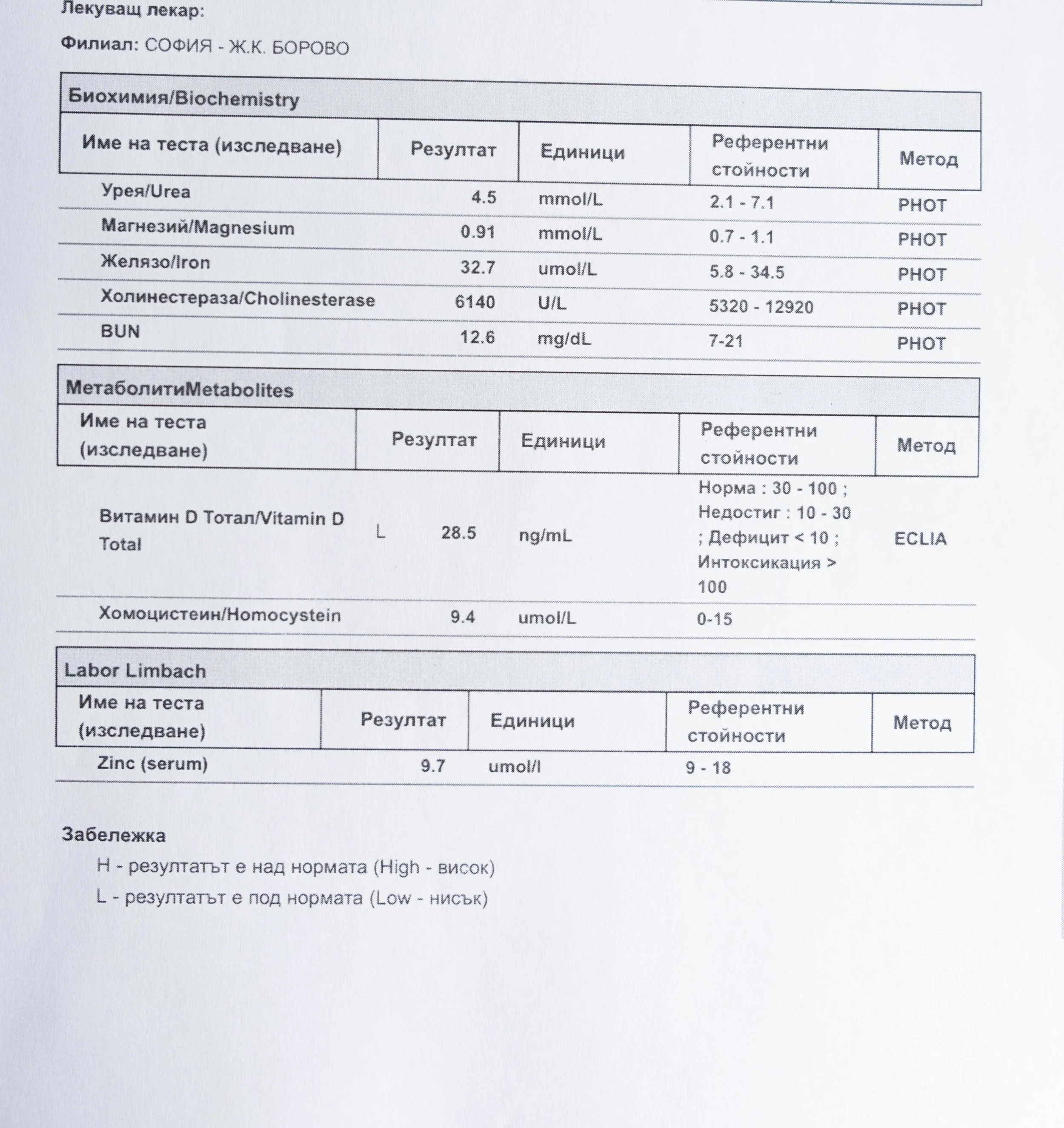
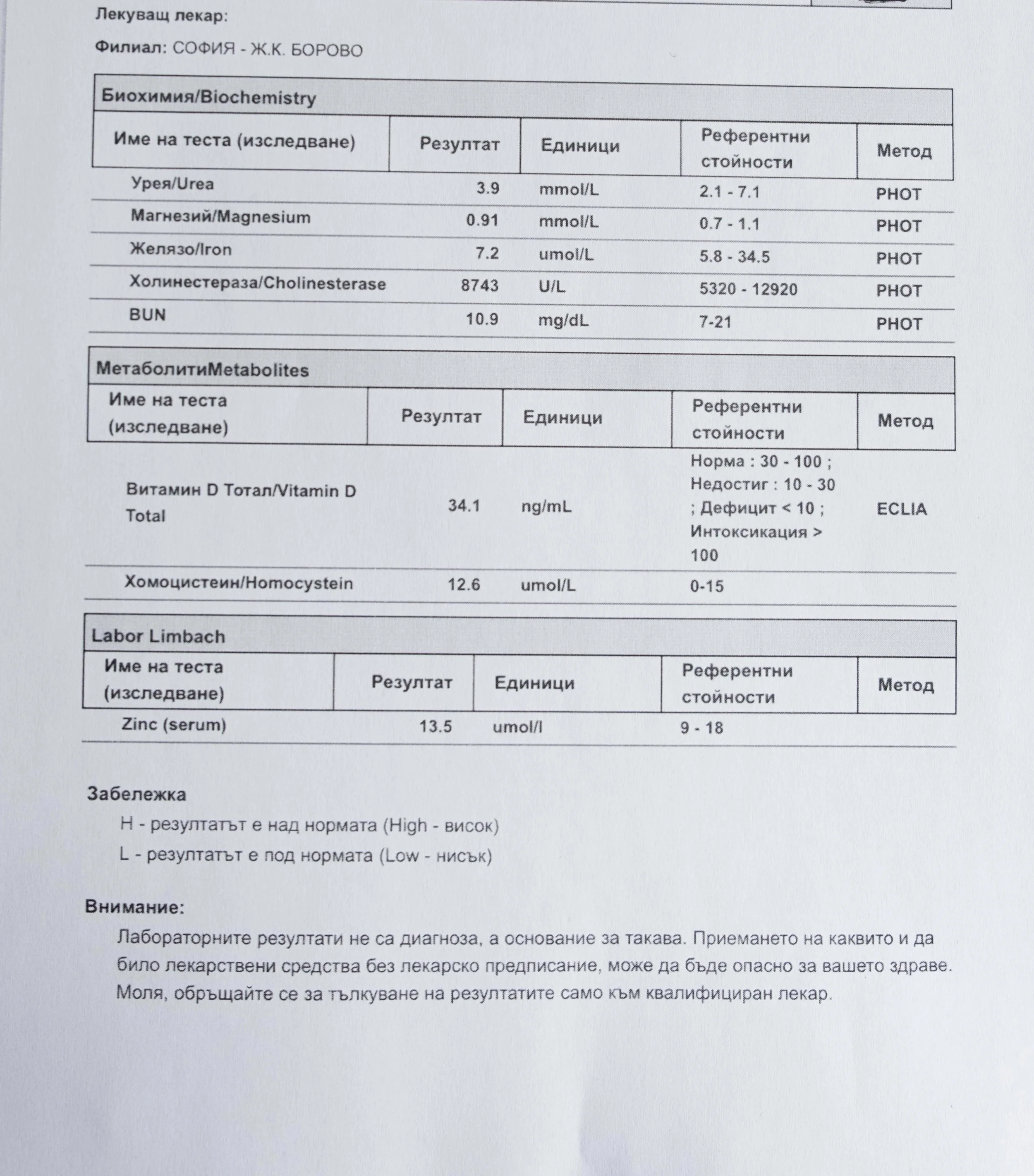
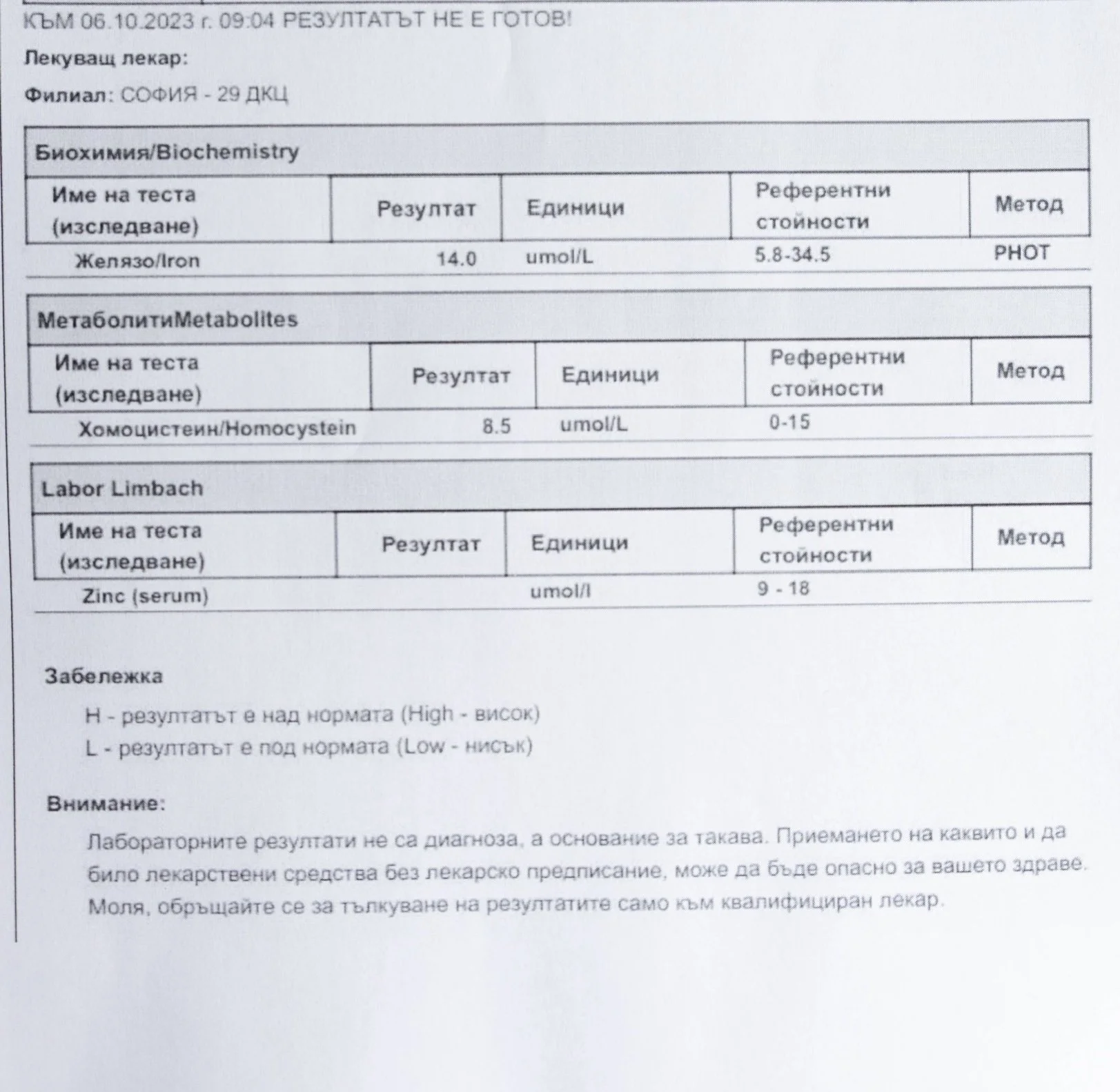
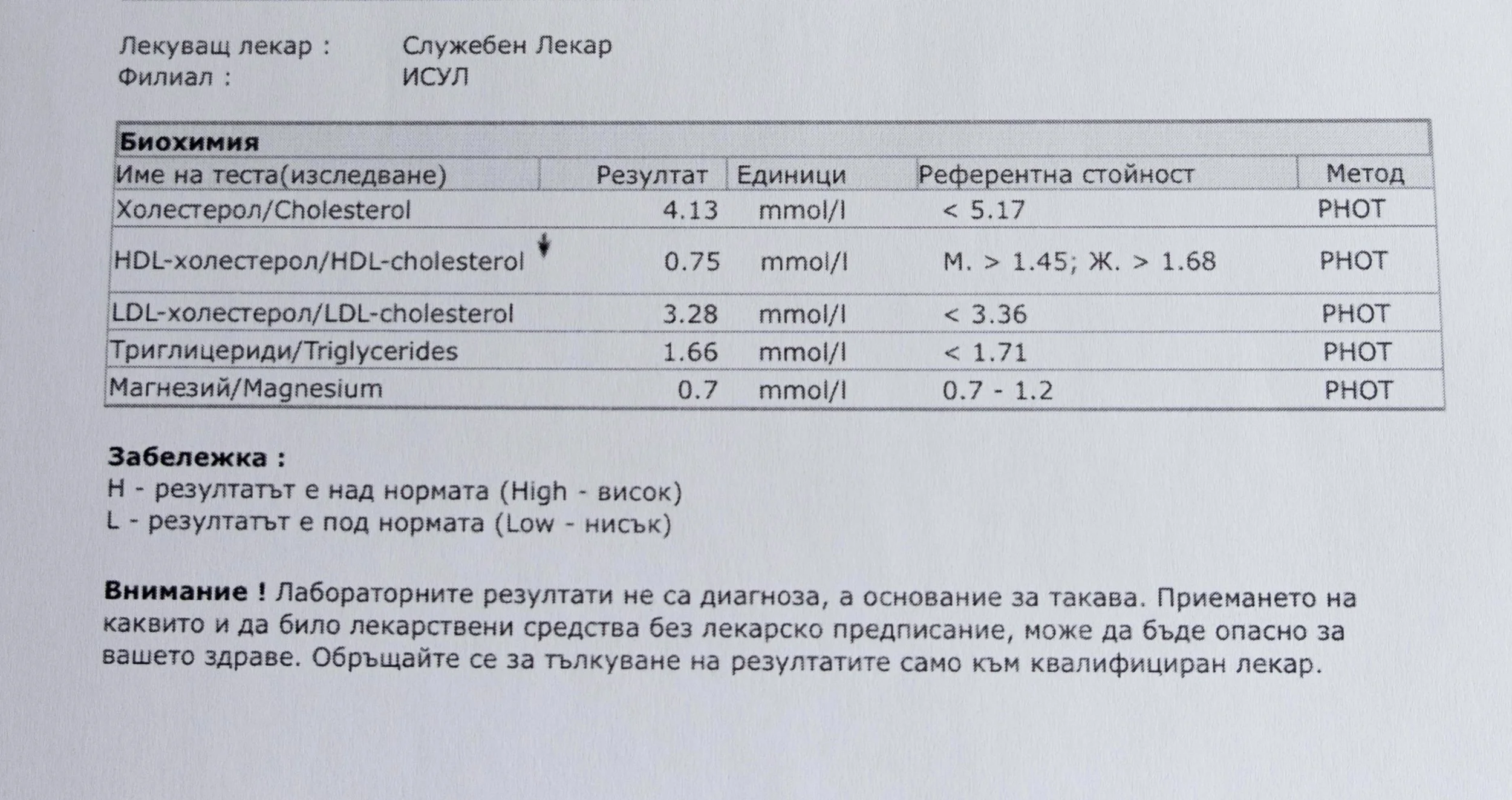
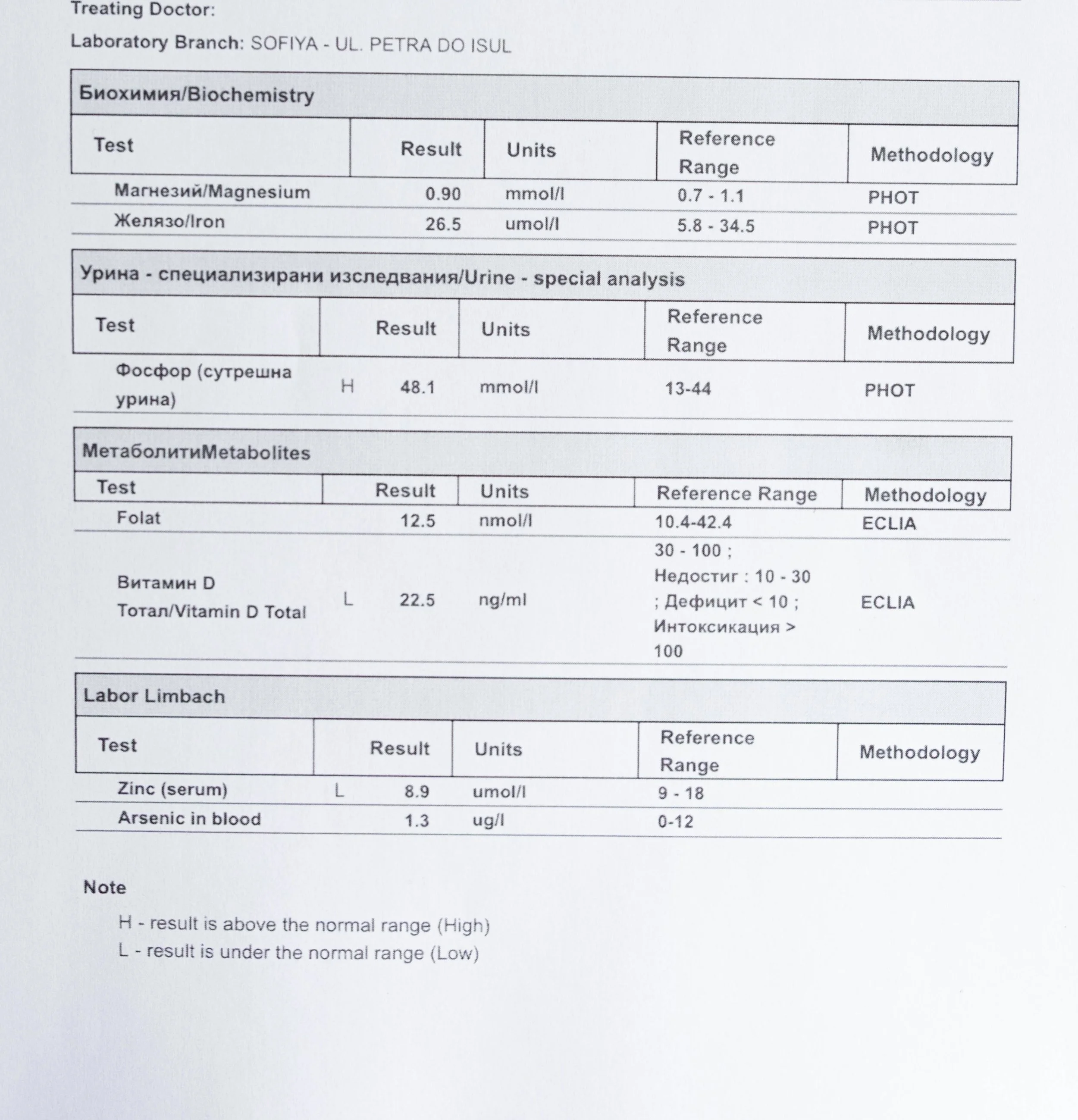
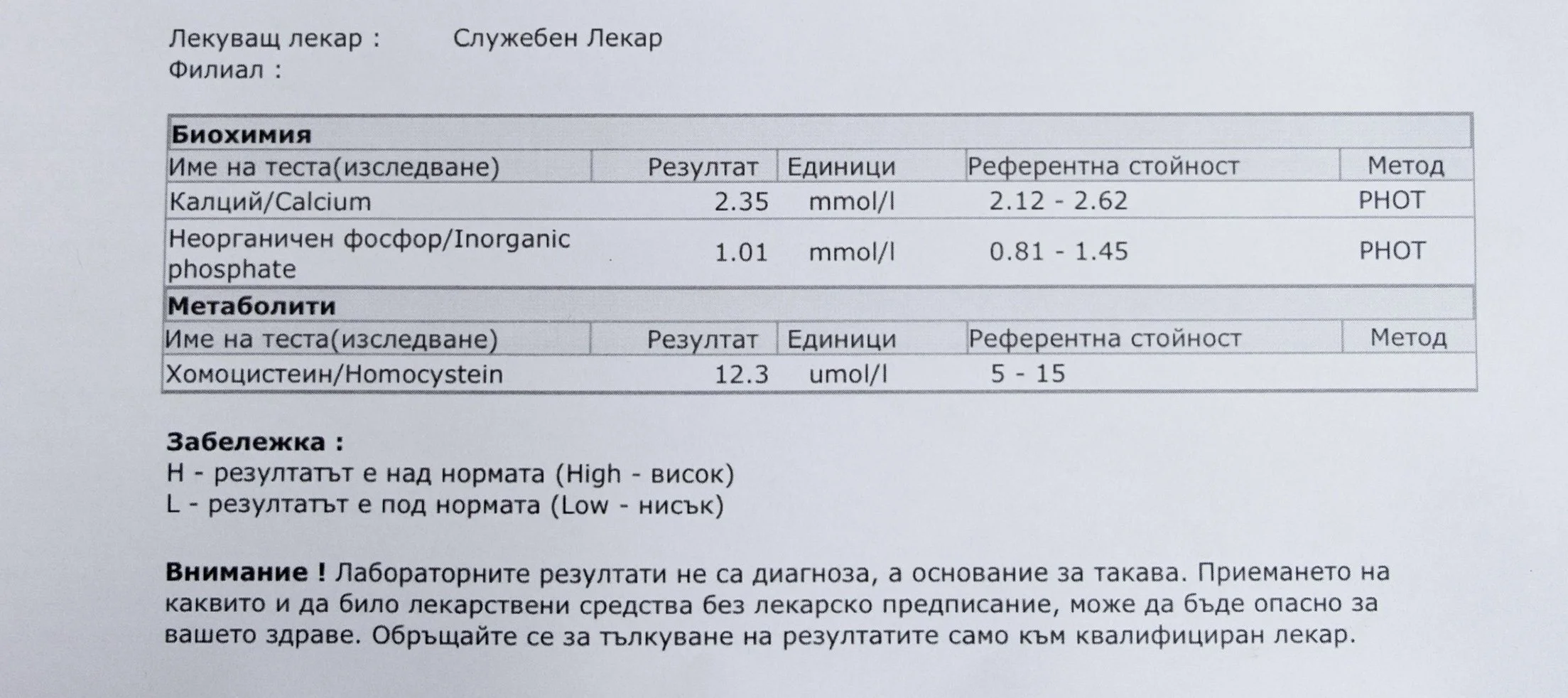
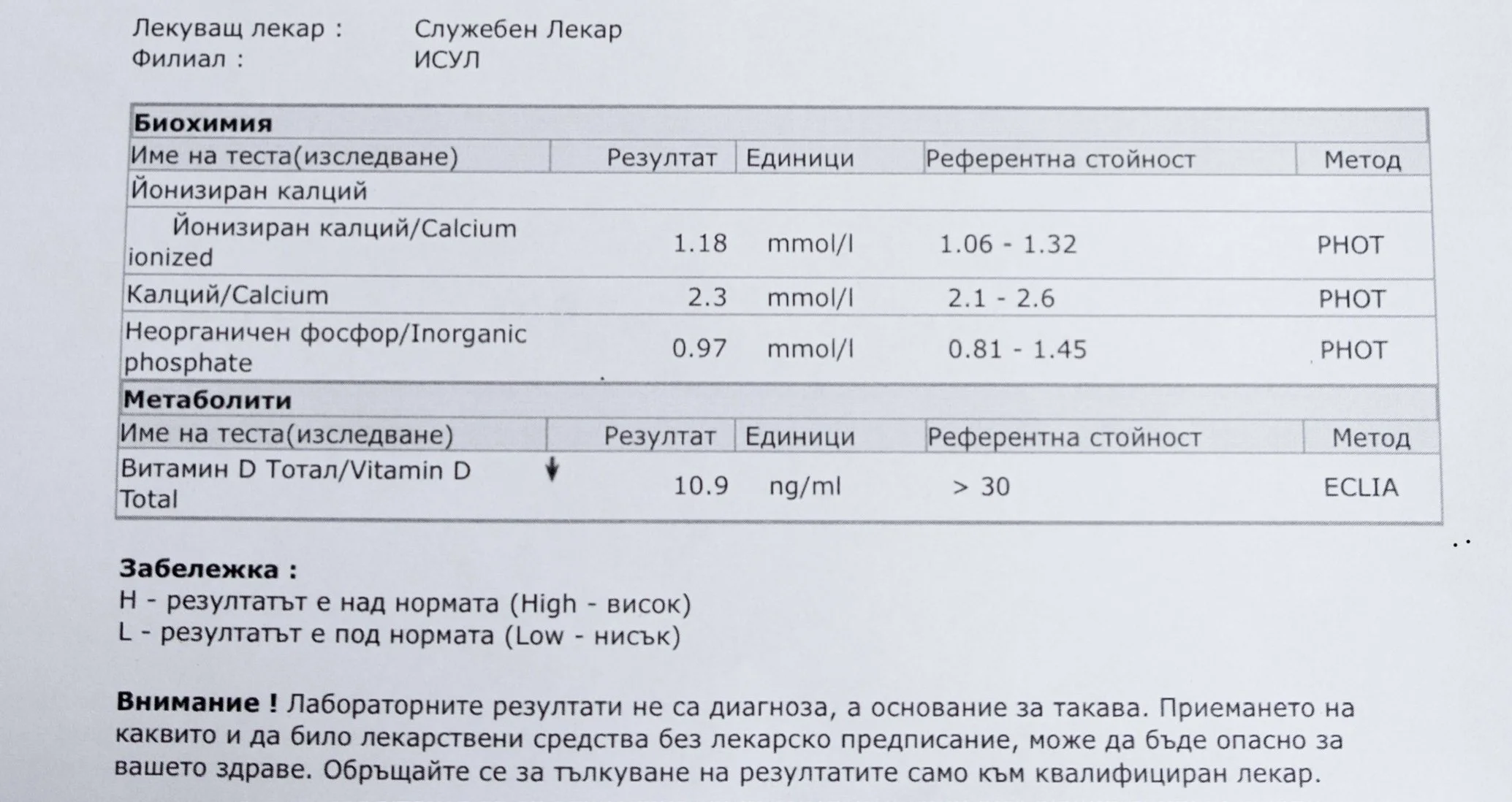
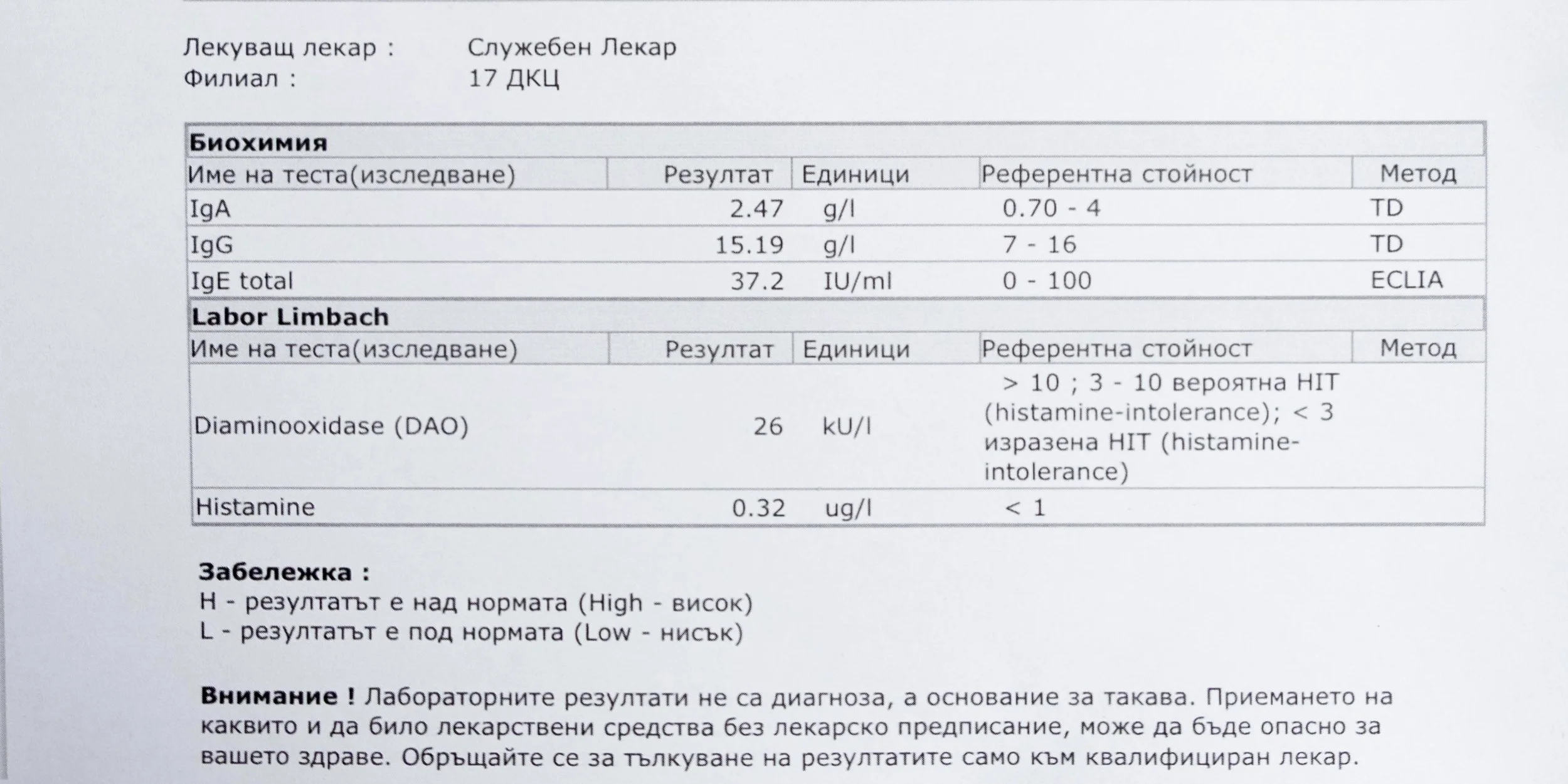
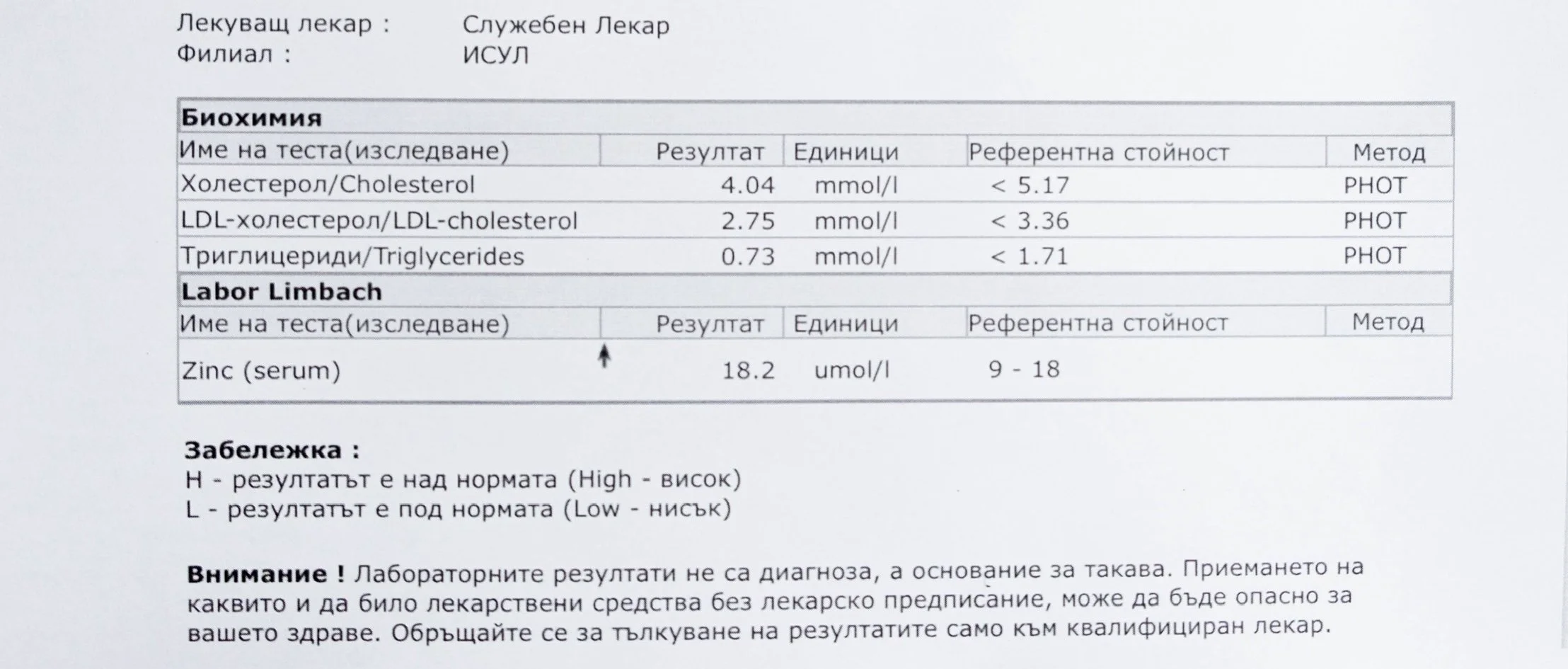
Repeated blood tests were conducted over several years, beginning with myself and later including other participants. These tests focused on markers related to mineral balance, one-carbon metabolism, and systemic regulation — including macrominerals and trace elements, vitamins involved in methylation pathways, homocysteine, and complementary biochemical indicators.
The purpose of this testing was not diagnosis, nor optimization. It was observation.
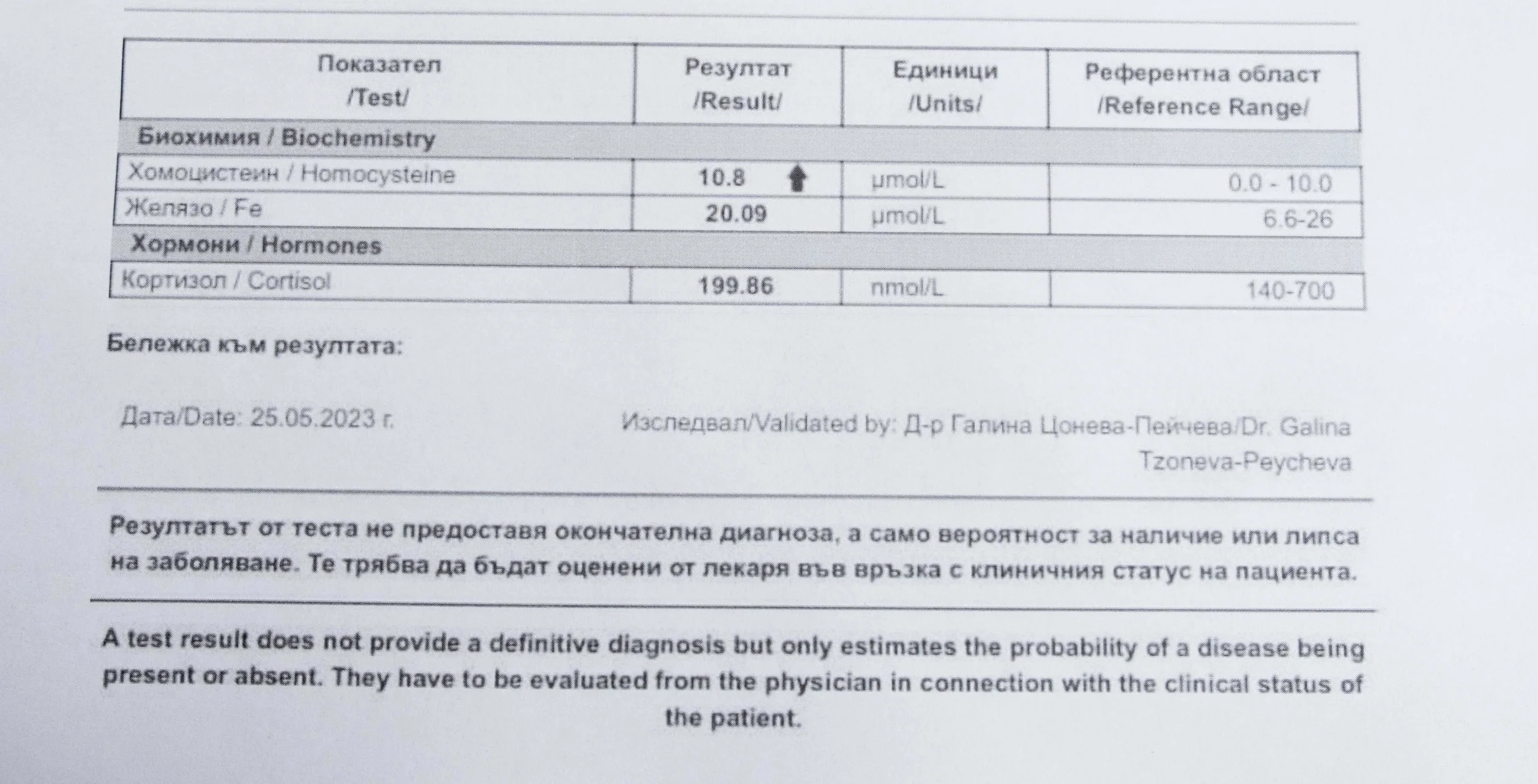
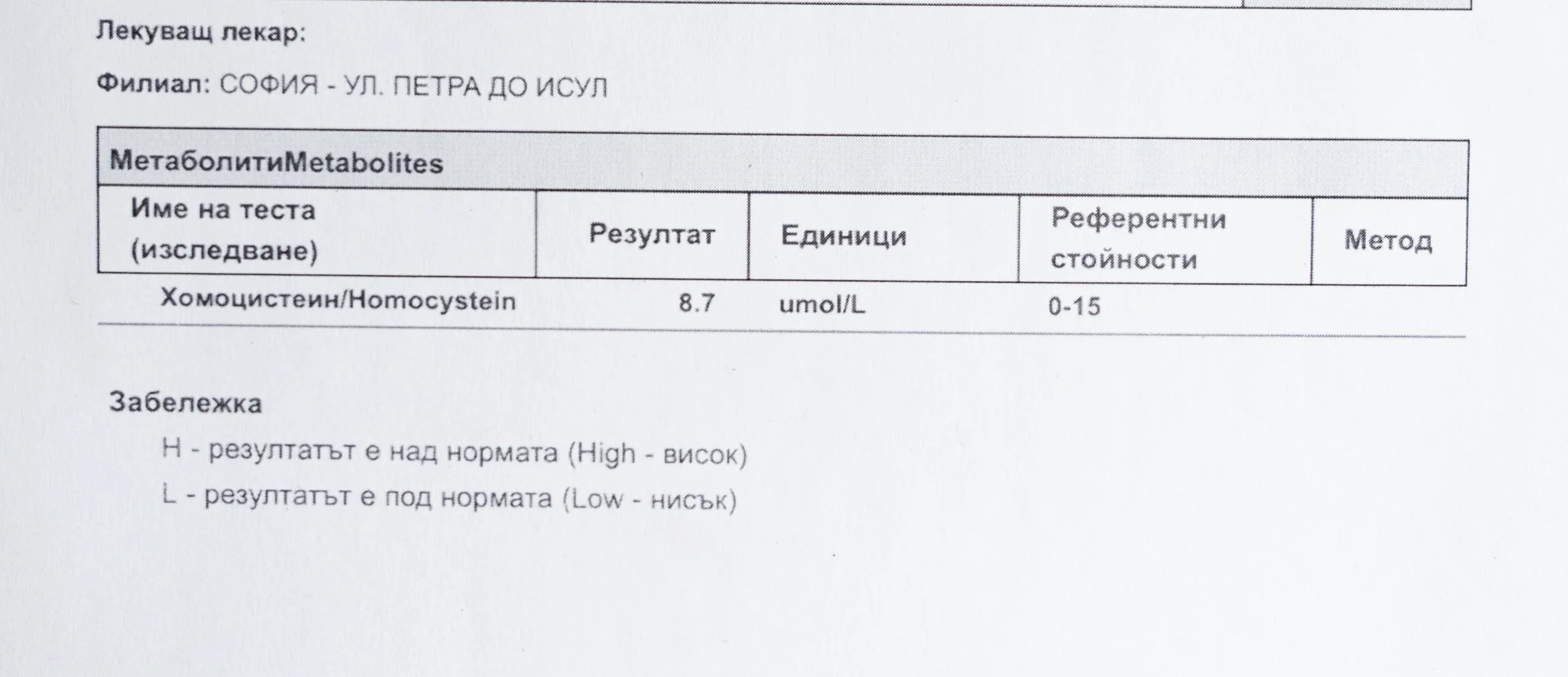
Laboratory data made it possible to track how prolonged stimulation and modern chemical exposure affect metabolic balance over time, especially where subtle shifts accumulate rather than presenting as acute deficiency or disease. In particular, homocysteine was used as a functional marker, because it reflects the integrity of one-carbon metabolism and the system’s capacity to complete methylation cycles.
Rather than relying on abstract models, the work required that biological claims remain visible in data: dates, markers, ranges, and patterns over time. This transparency allows the reader to see not only conclusions, but also the physiological context in which they emerged.
Why Minerals Matter in This Work
Most methylation research focuses on vitamins, because they are direct enzyme cofactors and easier to isolate in experimental settings. Minerals, by contrast, tend to operate quietly — stabilizing enzymes, shaping redox balance, and regulating ratios rather than driving single reactions.
Because mineral insufficiency often produces modulation rather than clear deficiency, its effects are harder to detect and easier to overlook. Yet without adequate mineral support, methylation processes may proceed unevenly, creating instability rather than resolution.
For this reason, mineral status was treated as foundational rather than secondary. Magnesium, zinc, copper, manganese and related elements were observed alongside vitamin markers — not as supplements to a model, but as structural conditions for biological timing and completion.
How to Read the Data
The laboratory tests presented here are not intended as clinical recommendations or normative standards. They are part of an investigative record — evidence of how biological systems respond over time under sustained stimulation and constraint.
Readers may use this material to:
understand the physiological basis of the work
trace specific biochemical relationships
verify claims against measurable markers
Detailed references supporting these observations are available in the Research Library.
Homocysteine as a Functional Marker of Stimulation
When one-carbon metabolism is impaired, its byproduct, homocysteine, rises, leading to hyperhomocysteinemia. This amino acid can be measured through a blood test at most laboratories. The acceptable levels of homocysteine have varied over time. In the past, hyperhomocysteinemia and elevated blood homocysteine levels were defined as levels above 15 umol/L, with moderate hyperhomocysteinemia defined as 16-30 umol/L, intermediate as 31-100 umol/L, and severe hyperhomocysteinemia as above 100 umol/L.
Visit: https://www.optimaldx.com/blog/homocysteine-optimal-range
Various studies have identified threshold values for homocysteine associated with increased health risks:
❍ 9.47 µmol/L – associated with increased risk of cardiovascular incidents
❍ 9.74 µmol/L – linked to short sleep duration
❍ 11.84 µmol/L – associated with increased all-cause mortality
❍ 15 µmol/L – significant predictor of cardiovascular events
A homocysteine level of 10 µmol/L or higher may be considered hyperhomocysteinemia, which requires further evaluation. Levels previously considered “normal,” with an average of 10.5 µmol/L, are now associated with the presence of atherosclerotic plaques in the carotid arteries of otherwise healthy individuals. Levels of 9.8 µmol/L or higher are linked to a 28% increased risk of cardiovascular disease.
According to Mosby’s guidelines, values below 4 µmol/L are considered low.
The optimal target range for homocysteine is 5 – 7.2 µmol/L.