Before reading the books, understand what problem they solve.
These six papers explain the biological condition that makes addiction inevitable in modern physiology. They prepare perception to see them.
If this layer is skipped, the books will be misunderstood as psychology.
These texts are not excerpts. They are the biological prerequisites required to recognize addictive energy as a structural phenomenon rather than a behavioral one.
The Source Is Not the Substance
Why different drugs produce the same unfinished state?
Addiction does not begin in the mind. It begins in where stimulation enters the body. Not all energy enters physiology the same way. Some signals can complete. Others cannot.
The Ancestral Chemical Conditions of Addiction
Addiction and control are not cultural accidents.
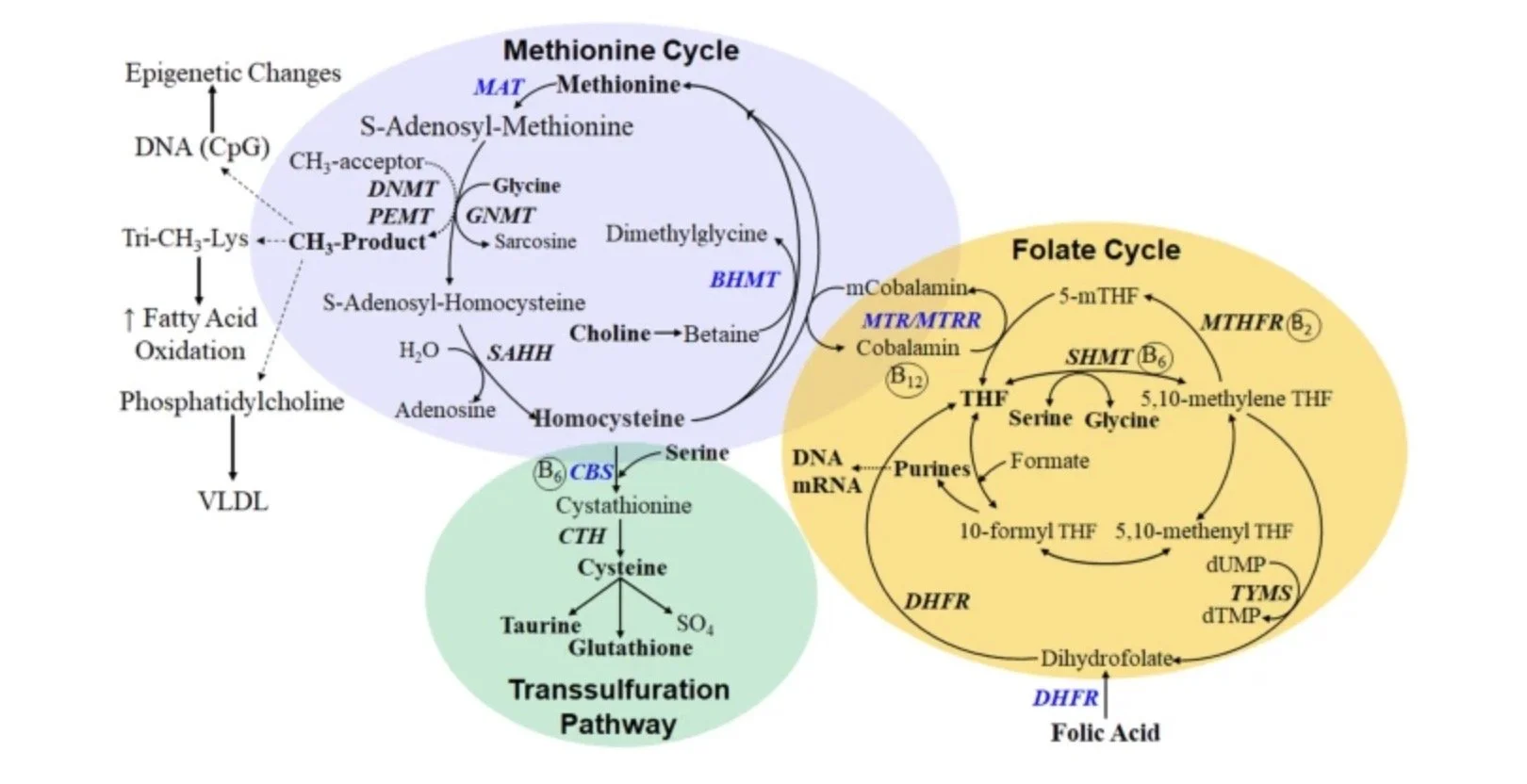
They arise from an ancient biological memory system built on methylation — a chemical mechanism that stores experience, regulates genes, and repeats stimulation.
Why Industrial Methylation Matters in Addiction?
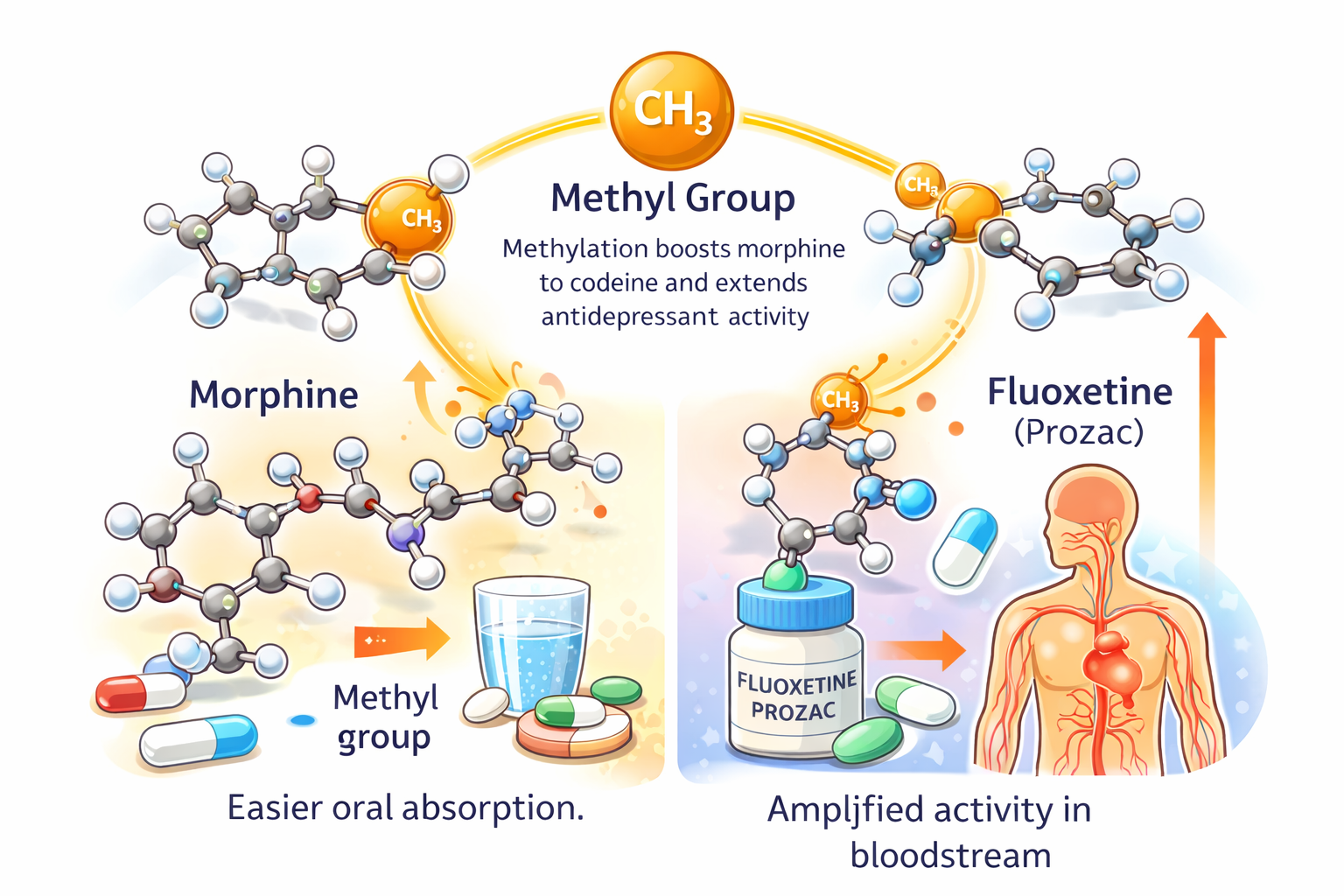
THE CHEMISTRY OF AMPLIFICATION
A single methyl group can make a molecule faster, stronger, and harder to release.
Industrial chemistry learned this early: methylated compounds cross the blood–brain barrier more easily, last longer in tissue, and intensify neurological impact. Small chemistry. Massive leverage.
Natural Methylation - the Biological Update System
Methylation continuously adjusts gene expression, metabolic timing, and neurotransmitter balance — including serotonin, dopamine, and histamine — in response to experience.
It is the mechanism through which stress, environment, and nutrition are translated into cellular change, allowing signals to resolve and the nervous system to return to baseline.
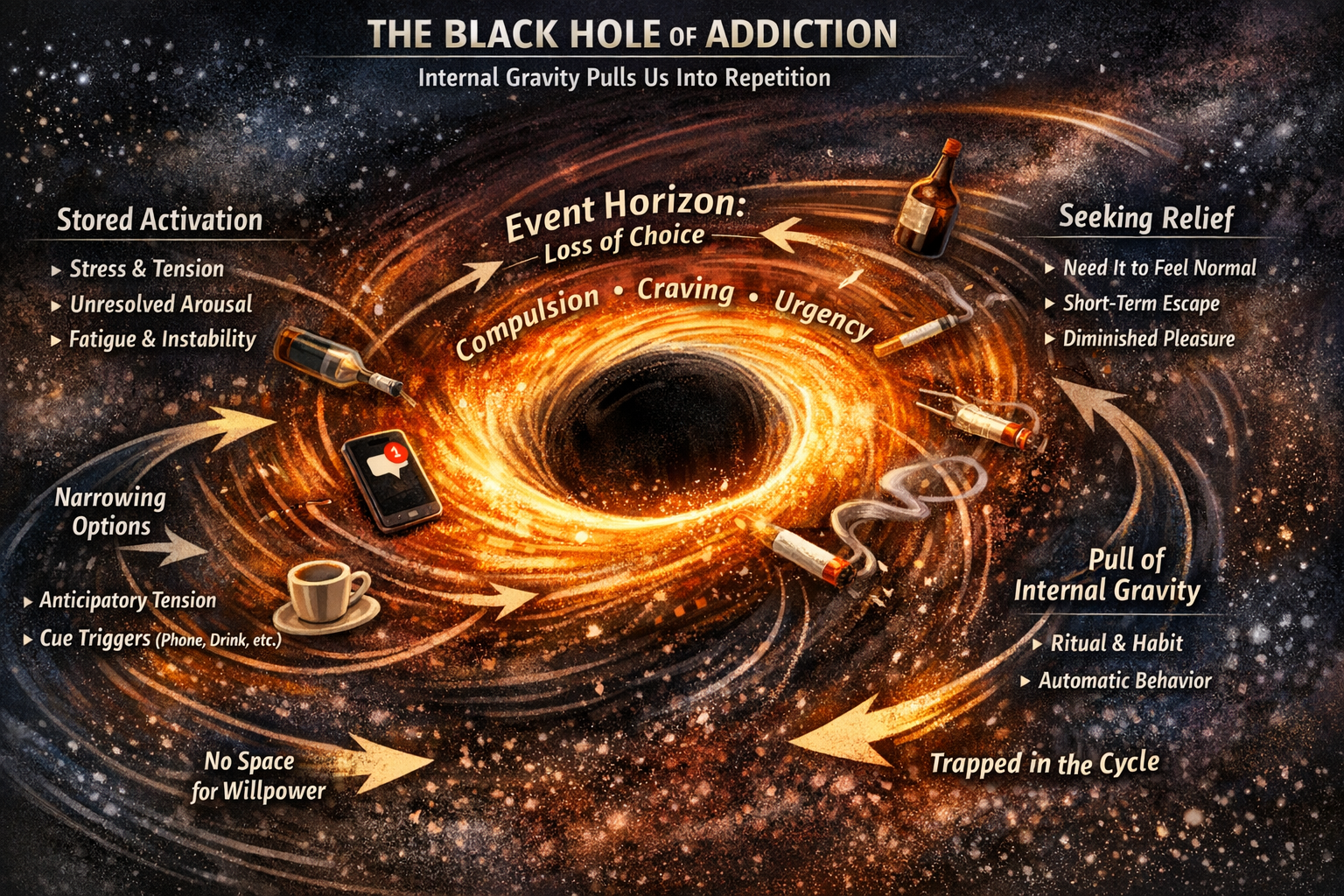
The Seven Phases of Addictive Energy
Addiction begins when a biological signal cannot complete — whether generated in the present or inherited from past generations.
From signal to civilization, the seven phases of addictive energy show how unfinished biology becomes modern structure.
By “internal gravity,” this work does not mean literal gravitational force like planets. It means a felt, directional pull inside the organism that emerges when activation builds up and cannot discharge.
It is “gravity” in the same way we say “a problem has gravity” or “I’m drawn to it” — but here the pull has a real biological basis.
If the mechanism described here is clear, the books will not read as theory.
They will read as recognition.